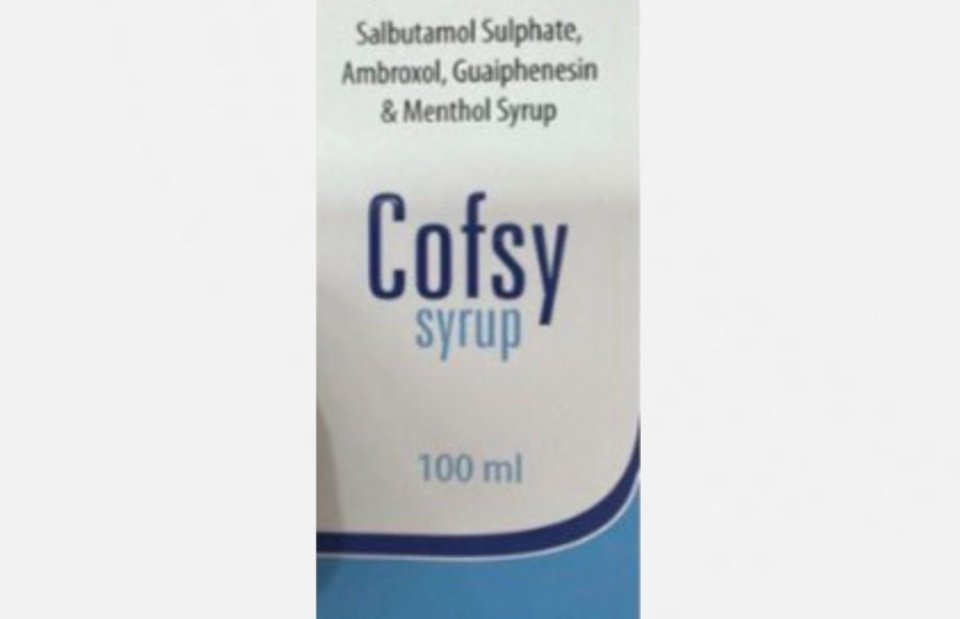
Maldives Food and Drug Authority MFDA has banned the use of cough syrup Cofsy amid reports of contamination.
In a statement, MFDA said that the syrup had been found to contain pieces of dirt or another type of contamination. It further said that it was conducting investigations to guarantee the medicine's safety.
MFDA said that until it had completed its investigation and published the probe results, the import, sale, and use of the medicine is banned in the Maldives.
The generic name for the medicine is Salbutamol Sulphate Bp + Ambroxol Hydrochloride Bp + Guiphenesin Bp + Menthol BP.
The cough syrup is being manufactured by an Indian company based in the Indian city of Chennai named Mmc Health Care Ltd.