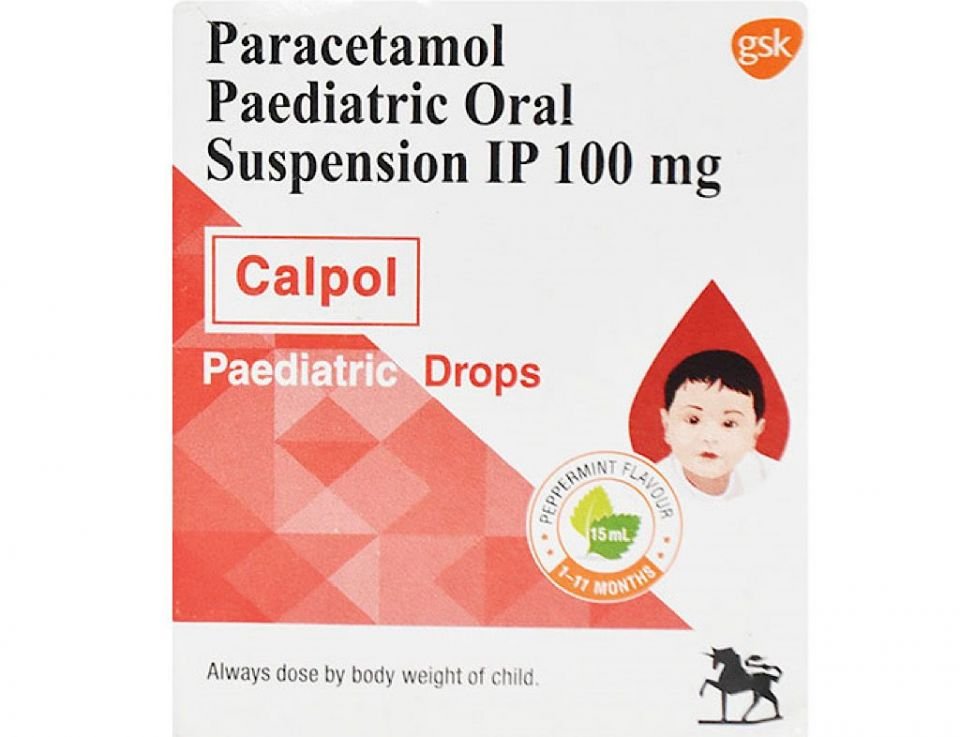
Maldives Food and Drug Authority MFDA has banned the use of a paracetamol medicine named Calpol: Paediatric Drops because of a quality issue.
MFDA said in a statement today that it had noticed quality issues from the batch of the medicine currently being circulated in the Maldivian market.
It said that the liquid medicine has been reported to have discolored and the medicine has been solidified in some cases.
The Authority further said that since it was carrying out further investigations, it had banned the import, sale, and use of the medicine in the Maldives. This medicine is manufactured by GlaxoSmithKline and is originally from India.
Earlier this week, MFDA banned the sale and use of 'Alergo Oral Liquid' (Cetrizine) as an MFDA random market sampling revealed that the medicine has been contaminated with DEG and EG. The medicine is manufactured by Pharmix Laboratories of Pakistan.